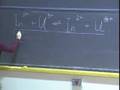Flash and JavaScript are required for this feature.
Download the video from iTunes U or the Internet Archive.
Topics covered: Discovery of Nucleus
Instructors/speakers: Prof. Sylvia Ceyer

Lecture 2: Discovery of Nuc...
Related Resources
The following content is provided by MIT OpenCourseWare under a Creative Commons license.
Additional information about our license and MIT You are not responsible for OpenCourseWare in general is available at ocw.mit.edu.
Great. Well, let's get going.
Last time we ended up by discovering the electron.
We discovered the fact that the atom was not the most basic constituent of matter. But in 1911 there was another discovery concerning the atom, and this is by Ernest Rutherford in England. And what Rutherford was interested in doing was studying the emission from the newly discovered radioactive elements such as radium.
And so he borrowed, or he got, from Marie Curie, some radium bromide. And radium bromide was known to emit something called alpha particles.
And they didn't really know what these alpha particles were.
Now, they did know that the alpha particles were heavy, they were charged and that they were pretty energetic.
That is what was known. Of course, today we know these alpha particles to be nothing other than helium with two electrons removed from the helium, helium double plus.
Rutherford is in the lab and has this radium bromide, alpha particles being emitted and has some kind of detector out here to detect those alpha particles.
And he measures a rate at which the alpha particles touch his detector. And it is about 132,000 alpha particles per minute. That's nice.
Then what he does is takes a piece of gold foil and puts it in between the radium bromide emitter and the detector.
And that gold foil is actually really very thin.
It is 2x10^-5 inches. Two orders of magnitude thinner than the diameter of your hair. I often wonder how he handled that, but he did it. He put it in the middle here and then went to count the count rate as a result of putting this foil there, and the count rate is 132,000 alpha particles per minute. It didn't seem like that gold foil did anything. The alpha particles were just going right through to the detector.
It didn't even seem to matter that there was that gold foil.
The post-doc that was working on it, Geiger, of the Geiger Counter, was actually disappointed.
Gee, that is a boring experiment.
But Geiger was even a little bit more unhappy because he had this undergraduate hanging around the lab, this undergraduate named Marsden.
And Marsden was really enthusiastic about doing something in the lab. He really wanted to do something. And Geiger, you know, what am I going to do with this kid?
Geiger goes to Rutherford, look, this kid really wants to do something. What should I have him do?
And Rutherford said, well, what you should have him do is take this detector and have him build it so that it can be swung around. So that it can be positioned here. So that we can check to see whether or not any of these alpha particles are backscattered, scattered back into the direction from which they came. And Geiger went away and thought, good, this is something to give the undergraduate. This is a ridiculous experiment. We know all the particles are going right through the detector.
Okay. But Marsden was real happy.
He gets to build this detector. He swings it around and gets Geiger there to do the first experiment.
He puts the radium bromide and they listen and hear tick, tick, tick, tick, tick, tick.
Geiger says, "Oh, it must just be background. Let me do a control experiment.
Let me take the gold foil out of here so that all the particles have to be going in this direction." They take the gold foil out of there and listen, and they hear nothing. They put the gold foil back and they hear tick, tick, tick, tick, tick, tick. And they put a platinum foil in there and they hear tick, tick, tick, tick, tick, tick. Whatever metal they put in there, there were some particles coming off.
And they got Rutherford down in the lab.
Rutherford looks them over their shoulder.
They do this again and again. Hey, it is real.
It is real. And what is coming off?
Well, the count rate is about 20 particles per minute.
Not large but not zero. And the probability here of this backscattering is simply the number of particles backscattered, which is 20, over the total number of particles, or actually the count rate that the particles backscattered over the total incident count rate. That is 2x10^-4.
That is not zero. Wow.
Rutherford was excited. Rutherford later wrote, "It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15 inch shell at a piece of tissue paper and it came back and hit you." What was the interpretation?
The interpretation was the gold atoms that make up this foil, they must be mostly empty. Now, they knew that those atoms had some electrons in it because the electron had already been discovered. But these alpha particles seem to be going right through those gold atoms, for the most part.
The atom, which he knew to be a diameter of about 10^-10 meters, most of that atom must be empty was the conclusion.
But occasionally these helium double plus ions, these alpha particles, hit something massive.
And that something massive then scatters those helium ions into the direction from which they came.
And since that probability is small, well, the size of this massive part has to be really pretty small.
And from knowing the probabilities and knowing roughly what the diameter of the atoms were and how many layers of atoms he had, he was able to back out of those experiments a diameter for this massive part of 10^-14 meters. And he called this massive part the nucleus. He called it the nucleus in analogy to the nucleus of a living cell.
The heavy part, the dense part in a living cell-- that is where the name "nucleus" comes from.
Now, Rutherford also realized that this nucleus here has to be positively charged. He knew about electrons and knew the atoms then were neutral, and so he reasoned this nucleus had to be positively charged.
And then he did a bunch more experiments, more sophisticated experiments in which he actually measured here the angular distribution of the helium ion scattered from the nucleus.
And from those very detailed measurements of the angular distribution, he was able to back out the fact that this nucleus, the charge on it was actually plus Z times e. Z is the atomic number.
e is the unit charge. He did a bunch of different metals and was able to establish that the nucleus had a charge of plus Z times e. His model is that there is a very dense center, 10^-14 meters.
This diameter of the nucleus is something that every MIT undergraduate should know. And he realized that then the electrons have to fill out the rest of this volume.
That was his interpretation from these results.
And think about Marsden, what a great UROP experiment.
He discovered the nucleus. Isn't that great?
Marsden had a long and successful career as a scientist also after that. Now, I should also tell you that this backscattering experiment is really the essence of how a quark was discovered. Quark are the fundamental elementary particles in protons and neutrons.
Essentially, they took a high energy particle, scattered it through the proton or the neutron, and it backscatters. And, in that way, they discovered the quark and measured the diameter of the quark. And this was done by a couple of my colleagues in the Physics Department.
Jerry Friedman and Henry Kendall, who has since passed away. Jerry Friedman is still around.
He loves to talk to undergraduates, and many of you will get that opportunity.
Now it is time for us to do our own Rutherford backscattering experiment. Yeah.
[APPLAUSE] Here is our gold lattice.
These Styrofoam balls are the gold nuclei.
The space around them are the electrons.
These things in the center here are just the posts on this frame. [LAUGHTER] This is a piece of equipment from my lab that I pressed into service, and so I couldn't cut these posts away because I would have trouble taking my manipulator out of my machine at a later time. So they are just there.
But this is our one monolayer of gold nuclei.
And so what are we going to do? Well, what we are going to do is try to measure the diameter of these Styrofoam balls in the same way that Rutherford did. And so we are going to need some alpha particles. What are we going to use for an alpha particle? Well, we have some ping-pong balls for alpha particles. Let's do that.
We have 287 alpha particles, or ping-pong balls, and we are going to measure the probability of backscattering.
The probability of backscattering will be the number that actually backscatter divided by the number that we throw, or the total number. That is what we are going to measure. But now I have to take this probability and I have to relate it to the diameter of these nuclei. How am I going to do that?
Well, that probability is going to be equal to the total surface area of the crystal here. I have already measured the total area. I know that the total area is 2,148 square inches. That is in the denominator, but now the numerator is simply the total area of the nuclei.
The total area of the nuclei is the area of one nucleus, A sub i, summed over the total number of nuclei, which I have already counted as 119.
And so the total area is times the cross-sectional area here of any one of these nuclei. And that is pi d squared over 4.
I can solve that equation, for the diameter, in terms of the probability.
And when I solve that equation, d is equal to 4.79 times the probability to the one-half power.
What we are going to do is measure this probability by throwing the ping-pong balls and calculating and determining how many backscatter. And then we are going to use that to get this diameter of the nuclei.
The same experiment that was done to actually measure the diameter of the nucleus. Now you are going to do this experiment. Every one of you are going to get a ping-pong ball from the TAs.
TAs, why don't you give out the ping-pong balls, and then I will give you some instructions.
All right. The pi d squared over 4 is the cross-sectional area in terms of the diameter of these balls. I just wrote it in terms of d instead of r. Yes?
That is correct. Good point.
That balls that we are throwing actually have size compared to in the case of the Rutherford backscattering experiment where the projectile was almost a point compared to the size of the nucleus. In our experiment, you are quite right, our balls are about the diameter there. And so, if we were doing a more exact experiment, we would do a little different calculation. We would take into consideration the size of the actual ball that we were throwing. But we are not going to do that. Because we are not throwing that many balls, we don't really have the statistics to do a more exacting kind of calculation.
But you are quite right. Yes?
Well, he didn't know. Although, he knew the fact that it was backscattering, that it had to be much, much less massive than the nucleus.
I think that he also measured the energy of the backscattered particle. And from that, you can back out the fact that it is much less massive than the nucleus. There are a few other details, you are quite right, that I have left out in this discussion that he had to know in order to get this number.
Here is the thing. You have to aim your alpha particles at this lattice. And then you have to watch your ball. [LAUGHTER] You have to watch to see if it scatters back at you, because at the end I am going to ask you if your ball backscattered.
And we need an accurate count. Now, if you hit one of these things and it backscatters, that doesn't count.
Only if it hits the Styrofoam ball does it count.
If it hits the Styrofoam ball and goes through, that doesn't count. It literally has to backscatter at you. Was there a question over here?
If you miss you miss. [LAUGHTER] Now, I do invite you to come a little closer so that you can at least hit the crystal. Yes?
That is correct. Well, you have got a defect.
These are a little bit lighter. Oh, you have some more here.
Oh, okay. You can have a regular one.
Anybody need one yet? I have a couple.
Oh, all right. You need one?
Because I need them all thrown. Did you have a question?
What is the mean free path?
That I am going to have to give you an expression for at some other time, but there is certainly a decay pathway.
I have another ball here. Now, are you ready?
You can come down closer, but now I have one piece of advice for you. That is, only fools aim for their chemistry professor. [LAUGHTER] Go to it.
Did you throw your balls? You missed the crystal.
All right. Has our supply of alpha particles been exhausted? All done?
All right. [APPLAUSE] Now comes the big test. How many of you had an alpha particle that backscattered? Let's keep your hand high because I have to count accurately.
In this section I see one. Two?
Cheater. No.
Two, three, four, five, six, seven, eight, nine, ten, eleven, twelve, thirteen. Did I get everybody?
I got everybody? 13?
Right, not deflection. If it hit and went through, that does not count. It has to come back at you.
Yes. [LAUGHTER] That is right.
All right. Does anybody want to change their count? 13 balls?
I am sorry? If it just hit it and moved but did not backscatter, it does not count.
The nuclei will move. They will move, certainly, because there is a momentum transfer.
Well, not quite like that. No.
We have 13 balls that backscattered?
Okay. Let's see what we got.
The probability, here, then, is 13 over 287.
That probability is equal to If I now that this probability and plug it into here, what we are going to get is a diameter of 1.0 inches.
And the diameter on the average of those particles is about 0.85 inches. You did a really pretty good job. You got the diameter of the nucleus. [APPLAUSE] That is great. And that is the way the nuclear diameter was, in fact, measured and discovered. But now we have the problem that the scientists had in 1912, and that is what is the structure of the atom? We now know it has a nucleus.
It has an electron. How do they hang together?
Where are they in the atom? We are going to talk about the classical description here of the atom.
And the first question that we have to ask is, what is the force that keeps the electron and the nucleus together? What are the four fundamental forces? Gravity is one.
And that is the strongest or the weakest?
Weakest. Gravity.
Next stronger force? Electromagnetic.
I will just abbreviate it EM. Next stronger force?
Weak force. And the next?
Strong. Weak and strong are intranuclear forces. They are operable between the protons, the neutrons and the other elementary particles that make up the nucleus. It does not have a lot of effect, the weak and the strong force, on chemistry, except for beta emission for the radioactive elements.
Gravity actually does have no known chemical significance to chemistry. And so all of chemistry is tied up here in the electromagnetic force, which I am, at the moment, going to simplify and just call the Coulomb force. Now, we know how to describe the Coulomb force between charged particles.
We know what expression to write down.
Let's do that. If we have the nucleus, which is positively charged, and the electron here, which is negatively charged, and they are at some distance r between each other, the expression that describes how that force of interaction changes with distance, this Coulomb's force law, it is just the magnitude of the charge of the electron times the magnitude of the charge on the nucleus over 4 pi epsilon nought times r squared.
I am going to just treat the force as a scalar, just for simplicity purposes here.
Epsilon nought is the permittivity of vacuum.
It is a factor in there for unit conversation.
r, then, is the distance between the electron and the nucleus. What does this say?
Well, this says that when r goes to infinity, what is the force? Zero.
The particles are infinitely far apart.
There is no force between them. In this case, an attractive force between them.
When r is equal to zero, what is the force?
Infinite. And anywhere in between, that force is described by this one over r squared dependence. You can see that as the particles come closer and closer together, the force between them gets larger and larger. The closer they get, the larger the force, the more they want to be together. This expression is just telling me, if I held one particle and the other particle in my hand, and I held them at some distance from each other -- That expression is just telling me the force with which I have to kind of exert to keep them apart.
But now, if I let them go, you know what is going to happen. They are going to come together. They are going to want to come together because of that force. And what is not in this expression? What is not in that expression is any information about how those particles move under influence of that force. Nowhere in this expression is there an r of t, how that distance changes with time. And so what we need to describe that is a force law. And in 1911, the force law that seemed to describe the motion of all bodies, including astronomical ones, of course, the equation of motion that described how bodies move are Newton's equations of motion. And, in particular, F equals ma. And, of course, I can write that acceleration as a time derivative of the velocity, dv over dt.
And that velocity, of course, itself is a change in the position with respect to time.
This is m, the second derivative of r with respect to time.
If I know the force that is operation, which is this, I can take this and plug it in here, and I am going to have a differential equation. And that differential equation is going to allow me to solve for what r is as a function of time, the distance between the two particles.
And it is going to allow me to solve for that distance in a way that we call deterministic, exactly.
In other words, if I know where the particles are to start with, using this equation of motion, this force law, I can tell you where those particles are going to be for all future time exactly.
It is deterministic, the classical mechanical approach. Now, in order to solve this differential equation, I am going to have to develop a model for the atom. All differential equations, for the most part, describing physical processes are going to need a model. They are going to need some boundary conditions or initial conditions.
And the model, of course, that came to mind for the atom, is one in which the nucleus is in the center. And the electron moves around that nucleus with uniform circular motion and with a fixed radius. We are going to call that fixed radius r star. It is a planetary model.
That seems like a good guess for the structure of the atom.
Now, if you have a particle undergoing uniform circular motion at some well-defined radius here.
That particle is being constantly accelerated.
And I can write that acceleration a as the linear velocity squared over that radius of its orbit.
It is being accelerated because the velocity vector. The direction is changing, so there is a constant acceleration.
Now, this expression, for many of you, I pulled out of the air. Some of you have seen it before. It is an 8.01 topic.
You are going to see it this semester, but later on and in this right now here, but you will recall later on this semester that you have seen it here in 5.112.
But, if this is the acceleration, I can take this expression for the acceleration and plug it into here. Plug in my operating force law.
And, in so doing, I am going to get -- -- e squared over 4 pi epsilon nought r star squared.
That is the F. Mass times the acceleration, m times v squared over r star. That is my equation of motion particular to this problem of a planetary model.
And now I can solve that for v squared, the linear velocity of that electron going around the nucleus.
That comes out to be e squared over 4 pi epsilon nought m r star.
Now, the reason I wanted to calculate the velocity squared here is because I want to calculate kinetic energy.
And that is easy to do. Kinetic energy, I will call K, is one-half m times v squared.
If I plug in the v squared right in there, I get one-half e squared over 4 pi epsilon nought r star.
So far, everything looks okay.
We have a planetary model. Coulomb's law is operable.
We know the acceleration. We just calculated the kinetic energy of this electron going around the nucleus.
What I want to do now is I want to know the total energy of the system. I just calculated the kinetic energy of the system, but I want to know the total energy of the system. And the total energy of the system, I am going to call this capital E, total energy, is the kinetic energy plus the potential energy.
And I want the total energy of the system for two reasons.
One is I want to show you that the system is bound, that the total energy is going to be negative, that it is lower than the total energy when the electron and the nucleus are separated. I want to show you that within this classical model, the electron and the nucleus do look bound. To do that, I need to show you the total energy is negative. To do that, I need to calculate the potential energy. That is what I want to do.
Secondly, I want to get an expression for the total energy.
Because, using that expression, I am going to show you how this classical mechanics fails. How Newton's equations of motion won't work to describe this problem.
Now, I have run out of time. I will do that on Monday, but that is where we are going. All right.
See you on Monday.
Free Downloads
Video
- iTunes U (MP4 - 83MB)
- Internet Archive (MP4 - 147MB)
Audio
- iTunes U (MP3 - 8MB)
Caption
- English-US (SRT)