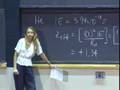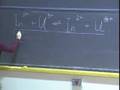Flash and JavaScript are required for this feature.
Download the video from iTunes U or the Internet Archive.
Topics covered: Internal Degrees of Freedom
Instructors/speakers: Prof. Sylvia Ceyer

Lecture 15: Internal Degree...
Related Resources
The following content is provided by MIT OpenCourseWare under a Creative Commons license.
Additional information about our license and MIT OpenCourseWare in general is available at ocw.mit.edu.
Last time we started talking about the internal motions of molecules, the vibrations and the rotations.
And we talked about how the different ways in which a molecule can store energy were called modes.
Or, sometimes called the degrees of freedom.
And so, in general, if we have a molecule that has N atoms, it has 3N degrees of freedom or 3N modes.
And we said last time, that three of those 3N modes are always translational modes. We live in a three-dimensional universe, so there are three translational modes, three directions in which the molecule can travel, can move. And, therefore, if we use three of these 3N modes for translation, then we have 3 minus 3 modes that are internal modes, internal degrees of freedom. And that is what we really want to start looking at today. On the diagram here, on the side, here is our N molecule.
There are 3N total modes. I just said three of them were translational modes. Now, how do we separate those remaining modes between rotation and vibration?
The way we do it this. If you have a molecule that is linear, you always have two rotational modes.
I will show you that in a moment.
Linear molecules always have two rotational modes.
Nonlinear molecules have three rotational modes.
Therefore, if we use, in a linear molecule, three of them for translation, two of them for rotation, the number of vibrational modes are what is left over.
For a linear molecule, we have 3N minus 5 vibrational modes. If we have a nonlinear molecule, since we have three rotational modes in a nonlinear molecule, we have leftover 3N minus 6 vibrational modes.
Now, let's take a look at this a little bit more in detail.
Let's start with nitrogen. Two atoms.
Six modes total. Three translational.
Two rotational modes of nitrogen.
What are those rotational modes?
Well, here is our nitrogen. And one of those rotational modes is going to be rotation about an axis in the plane of this board, and its rotation around this axis.
That axis goes through the center of mass of that molecule.
That is one of the rotational modes.
Another rotational mode is rotation around an axis, here, that is perpendicular to the board.
This is another rotational mode.
It is going to turn out that these two rotational modes are degenerate, meaning they are going to have the same frequency of rotation. They are going to have the same energy. We will talk about that in a moment. What I want to point out, here, is that if you look at the axis, here, in the plane of the board that is along the bond axis, this, hey, you know what, this is not a rotational mode because there is cylindrical symmetry around that bond axis.
It has no meaning, rotation around that bond axis.
The molecule looks the same for all of the angles from zero to 360 degrees. That is not a rotational mode.
Linear molecules, diatomics, you have two rotational modes. They are going to turn out to be degenerate, as we are going to see.
And then, our table over there says for nitrogen, we ought to have one vibrational mode.
And we do. That one vibrational mode, here, is the nitrogen-nitrogen stretch.
It is a stretch mode. We said last time that these bonds function like a spring. The molecule can stretch, the molecule can compress. We are going to look at that in more detail in just a moment. Next, in our table over here, let's look at CO two. CO two has three atoms.
Nine total modes. Three of them are translation.
CO two, even though it has three atoms in it, is a linear molecule, so it has only two rotational modes. Let's look and see what those two rotational modes are. One of those rotational modes is, again, rotation around an axis in the plane of the board, here, around the center of mass of that molecule.
That is one rotational mode. The other rotational mode is around an axis perpendicular to the board here.
That is a second rotational mode.
These two modes are going to be degenerate because they are actually the same motion, just in a different plane.
These are degenerate. And then, again, just to emphasize, if you think that you have a rotation here along that linear bond axis, well, the answer is no, this is not a rotational mode.
So, we have two rotational modes for CO two.
And now what does our table say in terms of vibration?
Well, in terms of vibration for a linear molecule, 3N minus 5, we have used now five modes.
We have four left, 3N minus 5.
What are those modes?
One of these modes is what we call a symmetric stretch.
A symmetric stretch means that this oxygen and this oxygen are simultaneously stretching or are simultaneously compressing.
My body is the carbon, and my arms are the oxygen.
A symmetric stretch is the oxygens both moving in or both moving out. That is one vibrational mode, this symmetric stretch. That is one vibrational mode.
What is another vibrational mode?
Well, if you have a symmetric stretch, it must mean you have an anti-symmetric stretch.
In this case, we have one oxygen moving out while the other oxygen is moving in.
And this carbon is kind of moving in like that, too. Or, this one is moving in, this one is moving in, this one is moving out.
That is the anti-symmetric stretch.
If my body is the carbon and my arms are the oxygen, this is the anti-symmetric stretch with the carbon moving just a little bit to the side, too.
The anti-symmetric stretch, another mode.
These two modes are not degenerate.
They have different frequencies.
They have different energies. We will see that in a moment.
What are the other modes? Well, the other modes are a bending mode. What can happen is that this oxygen and this oxygen can move in, and this carbon will kind of move out. Or, of course, the other way, here.
These go like that, this goes like that, or that goes like that. This is a bending vibration.
Again, if my body is the carbon and these are the oxygens, the bending vibration looks like this.
That is the bending vibration. However, you could also imagine that you have a bend in another plane.
In other words, you can imagine that these two oxygens here are coming out at you or going in at you.
And so, in other words, if I am the carbon, these are the oxygens. We also have a bend like this.
These two bending vibrations are degenerate.
These are four different vibrations.
They are the four vibrational modes, two bending, one symmetric stretch, and the other, the anti-symmetric stretch. That is what we have in CO two. What about another molecule?
How about water? We have three atoms.
Again, nine total modes. Three of them are translation, but now water is not a linear molecular.
So, we are going to have three rotational modes in the case of water. Let's look at what those three rotational modes ought to look like.
Here is our water molecule. One of those rotations is, again, a rotation around an axis here in the plane of the board. That is one rotational mode.
If I am the oxygen and my hands are the hydrogen, this is a rotational mode. You got that?
Okay. And then another rotational mode, here. It can be a rotation around the center of the mass, centered on the oxygen here.
Axis perpendicular to the plane of the board.
This is that rotation. And I am sorry, but I cannot do cartwheels. That is one rotational mode.
And then a final rotational mode involves rotation, now, around this axis. It is an axis perpendicular to this one, but in the plane of the board.
And this is a rotational mode. We have three rotational modes.
This one requires me to do some flips, which I am also not going to do. But each one of these modes has a different frequency, has a different energy.
And you can see we have three of them now because we no longer have a linear molecule. We have three rotational modes.
Now, what about the vibrations for water?
Well, you can see from our graph up here that 3N minus 6, in this case for water, is going to be three vibrational modes. Let's look at that.
One of those vibrational modes is a symmetric stretch, again. The hydrogen is moving in or both moving out at the same time.
A picture of the symmetric stretch is, again, I am the oxygen, arms are the hydrogen.
This is the symmetric stretch, right?
And then, if there is a symmetric stretch, there is going to be an anti-symmetric stretch.
What that means is one of the hydrogens is moving in and the other one moving out. Or, this way.
Of course, a picture for that is this kind of a motion.
You get the idea. Anti-symmetric stretch.
And then, finally, we have a bending mode.
A bending mode, meaning these hydrogens moving this way or those hydrogens moving that way.
And, of course, a picture for that is this.
That is a bending mode for the hydrogens.
We have three vibrational modes for the hydrogen.
Now we are going to look at some of the principles behind these internal degrees of freedom.
But, of course, the other reason for telling you about this-- Did you have a question?
It can, but it is not a separate mode.
That is correct. That is right.
It looks like a rotation, actually.
That is absolutely correct. The other reason for talking about this is to tell you that each one of these vibrations or rotations occurs at a frequency at an energy that is characteristic of the molecule. If we had a way to actually measure the frequency of these vibrations or the frequencies of the rotations, well, then we have a great tool for identifying what kind of molecule we have.
Analytically, this is a wonderful technique for identifying, ultimately, the molecule that you have, if you can measure those frequencies of vibration or rotation. And we are going to do that in just a moment. But what we also have to understand, before we go and measure these frequencies, is we have to understand that the energies with which a molecule vibrates or rotates are quantized.
So, that is what we have to spend some time thinking about right now. Let's do that.
And we are going to start with vibration.
To illustrate this, I am going to draw one of these energies of interaction, again, one of these curves that we have been talking about. And I am going to set my zero of energy at the dissociated atom limit.
So, I am going to talk about H plus Cl.
And, of course, right here, we talked about this being the equilibrium bond length, r sub e, for the HCl molecule.
That is the energy of interaction.
I am going to now draw in the ground vibrational state of HCl, which I am going to represent as a line.
It is going to be the energy. There is the ground vibrational state of HCl. The ground vibrational state, that energy is characterized by a quantum number.
The quantum number is the vibrational quantum number for the ground vibrational state. The vibrational quantum number is v equal zero. What this line represents is the ground vibrational energy. It also represents the extent to which the molecule's bond stretches and compresses.
The bottom line, here, is that the bond stretches up to here. The inner section of this line with the interaction energy, here, represents the maximum distance of the bond, the maximum bond length, the most that the bond stretches.
r is going this way. The intersection of this energy with this curve represents the closest that the two nuclei get.
This represents the most that the molecule has compressed.
This is the smallest value of r.
That is what that diagram represents.
If you start out, here, with an HCl molecule at the equilibrium bond length, right here, what happens is that the HCl will stretch, and it will stretch to this position. This is an exaggeration.
This is how much it stretches. And then, it comes back and goes through the equilibrium position.
Over here, and this is another exaggeration, this is how close they get to each other.
This is how much they compress. This point is often called the inner turning point, inner because it is the smallest bond distance. This is often called the outer turning point because it is the larger bond distance.
It is outer because it is at this distance, now, that the bond turns around and begins to compress.
This HCl molecule here is vibrating, equilibrium position.
Then it goes to the maximum extension, comes back through the equilibrium position, and then goes to the maximum compression, comes back to the equilibrium position.
That is what this line literally represents.
It is also the energy, which I will explain a little bit more in just a moment. So, this molecule is vibrating from here to here to here and back and forth.
Now, what I also want to point out to you is something that I have been kind of misleading you about for the last few weeks.
That is that this molecule is not sitting in the bottom of this well. Remember that when we were drawing bond association energies, I was drawing it from the bottom of this well to the dissociated atom limit?
I told you this was delta E sub d.
Well, the bottom line is that it is not really correct.
The reason is, is because the molecule is never really sitting at the bottom of this well.
It is sitting this much above. It is sitting at the v equal zero state. It is not sitting at the bottom of the well. If it were, it would not be vibrating. If it is not vibration, it will violate the Uncertainty Principle, something we didn't talk about. But the bottom line is that it cannot sit at the bottom of the well.
It is always vibrating by about this much energy.
When you have an experimentally determined dissociation energy for the bond energy, the experimentally determined energy is actually the energy from this level.
From v equal zero up to this dissociation limit.
This is the experimental bond energy.
Because, if you are going to measure a bond energy in the laboratory, well, you can only measure it from the lowest level at which that molecule can possibly be at.
It is not at the bottom of the well.
The difference between where this level is and the bottom of the well actually has a name. That difference is called the zero point energy.
And I will explain that in a moment, when we look at the energies a little more carefully.
Now, just for completeness, and this is not something that you have to know, let me tell you what the nomenclature usually is here for these well depths.
The nomenclature from the bottom of the well to here, which is not experimentally measured, is usually D sub e, dissociation sub e.
From the v equal zero level to the dissociation limit, that energy here is D sub zero.
You don't have to know these, but I just want to tell you, when you see that. All dissociation energies experimentally measured are measured from here to here because that is the lowest energy the molecule can have, is being in the v equal zero ground vibrational state. Well, now let's take a look at the energies a little more carefully.
Here is our favorite diagram again, our intermolecular interaction potential. This is HCl, so here is the dissociated atom limit.
This is v equal zero, right in there.
Now, there is an excited state for the HCl, or for any molecule. v equal one is the first vibrationally excited state. v equal two is the second vibrationally excited state.
v equal three is the third.
v equal four is the fourth.
v equal five is the fifth.
These are the allowed vibrational energies.
In other words, the molecule can vibrate with this energy or this energy or this energy, but not some arbitrary energy, like right in here or like right in there. These are the allowed vibrational states. And we have a nice analytical expression to describe those energies.
That analytical expression is right here.
That energy is h times nu times (that vibrational quantum number plus one-half).
So, v is that vibrational quantum number.
This nu, here, is the fundamental frequency, which is the frequency with which the molecule vibrates.
It is the frequency with which that molecule stretches, comes back to the equilibrium position, compresses, and comes back to the equilibrium position.
It is the number of cycles per second that molecule makes.
So, nu is the number of cycles, here, that the molecule in v equal zero makes per second.
Stretches, compresses, and back to the equilibrium position. Notice it is also the vibrational frequency of v equal one.
The vibrational frequency does not depend on the vibrational quantum number. You see no dependence of the vibrational quantum number on the frequency.
v equal two vibrates with the same frequency.
v equal three. v equal four.
v equal five. All of these states have the same nu here, have the same fundamental frequency. What does that mean?
That looks a little strange in the sense that if you have a molecule in v equals zero, the number of cycles per second that it makes is the same as a molecule in v equal five.
Well, you can see that in v equal five, the molecule has to stretch further, and it has to compress further.
It has to travel more distance. And so, if it has to travel more distance, but it still carries out the number of cycles per unit time, the same as v equal zero, well, then it must mean that the molecule in v equal five is moving more quickly, is moving faster.
And that is what that means. The energy of this state is higher. It is moving faster.
But it is moving with the same frequency nu.
That is the fundamental frequency.
That fundamental frequency, of course, is in hertz, our unit for all frequencies here.
Now, I drew on the board and called this energy from the bottom of the well to v equal zero, the zero point energy.
That is what it is. But numerically what that energy is, is one-half h nu. Stick in v equal zero, you get one-half h nu.
This energy, here, is measured from the bottom of this well. It is important that you understand where this energy is measured from.
It is measured from the bottom of this well.
v equal one, you put that in here and get three-halves h nu.
v equal two, you get five-halves h nu.
v equal three, you get seven-halves h nu.
The other thing to notice is that these states are equally spaced. The energy spacing between any two adjacent states is h times nu.
That is incorrect. What I am drawing, here, is right. I am sorry about that.
One-half h nu is from the bottom of the well to v equal zero. I think I know what happened.
What I have shown you here is correct.
What is in your notes is printed incorrectly.
You should make note of that and change that.
Suppose we have a molecule in the v equal zero state, where the energy is one-half h nu, that molecule can be excited to the v equal one state.
That molecule in v equal zero is still vibrating, but we can make it vibrate even faster, with more energy.
The same cycles per second, but with more energy, by promoting it to v equal one. We can do that if the molecule absorbs a photon. And the energy of that photon has to be equal to the difference in the energies of these two states. So, if the final energy, here, of the v equal one state, that is the final state, if that energy is three-halves h nu and the energy of the initial state was one-half h nu, then the difference in energy between the states is h nu.
We have to have a photon that has exactly this energy different, delta E. That delta E, then, has to be gotten by a photon with a frequency equal to nu. And the frequency of that photon that is going to make that transition is actually also the fundamental frequency of the molecule.
We are lucky on this one. Nu is delta E over h.
We have to have a photon with that frequency for the molecule to be promoted from v equal zero to v equal one. How do we know what that is?
Well, we do an experiment. We do an infrared spectroscopy type of experiment. The difference in the energies between vibrational states is in the infrared range of the electromagnetic spectrum. And so we are going to use infrared radiation. We have some source of infrared radiation. It puts out all different frequencies in the infrared. And then we send it through a monochromator. The monochromator disperses that radiation in space and allows only a certain frequency of radiation to come right through the slits and out into our sample. It is like you had the diffraction glasses, where we disbursed the radiation in space. Well, a monochromator is essentially the same thing. There is a diffraction grading in it. It allows, out of the front end, only radiation of a certain frequency to come out.
And we can adjust what that radiation is or what frequency that radiation has. We then allow it to pass through our sample, say HCl.
And then there is photo detector over here, some kind of photomultiplier. And what will happen is that if this molecule is going to absorb frequency of that radiation, well, then the number of photons reaching the photo detector is going to go down. If the frequency does not match the frequency of the vibration of HCl, well, the photons go right through, onto the detector.
But if it matches, then the molecule absorbs those photons and they never make it to the detector.
So, a plot looks like this. This is the intensity at the detector versus the frequency of the radiation.
The intensity will be high, but now at some frequency, which will turn out to be the vibrational frequency of the molecule, the intensity goes down low.
And then, as you go to higher frequency, the intensity comes back up. Right here, the molecule has made the transition from v equal zero to v equal one because the frequency of that photon matches the energy difference between v equal zero and v equal one divided by h.
It also happens to be the fundamental frequency of the molecule for HCl. So, that is how we know what those fundamental frequencies are.
Let's now look at what those energies are.
We have gone from v equal zero to v equal one.
Delta E equal h nu. We just measured nu as 8.6x10^13 inverse seconds.
Multiply it by h. That is going to give us delta E. The difference in energy between the two states is 5.7x10^-20 joules.
If I turn that into kilojoules per mole, that is 35 kilojoules per mole. That is the energy difference from here to here, about 35 kilojoules per mole.
How strong is the HCl bond? Well, the HCl bond from here to here is about 420 kilojoules per mole.
This is about a tenth, a little bit less than a tenth of the total bond strength for HCl.
If we make it vibrate, we are not going to make that bond break. It is an order of magnitude less energy here than what you need to break that bond.
Now, here is something that is a little confusing.
These fundamental frequencies, it turns out that we actually use a different convention to label them, different units.
We do not use Hertz very often to talk about the fundamental frequencies of molecules. We use something called a wavenumber instead of hertz. A wavenumber is an inverse centimeter, centimeter to the minus one power.
The symbol for a wavenumber is a frequency sign with a bar on top of it. That is a wave number.
How do we get a wavenumber? Well, we get a wavenumber by taking the frequency in Hertz, inverse seconds, and dividing it by the speed of light.
But the speed of light, of course, has to be in centimeters per second. The seconds cancel, and what we have then left is inverse centimeters.
For example, if the fundamental frequency of HCl was 8.6x10^13 hertz, we divide that by the speed of light in centimeters per second and the wavenumbers, or the fundamental frequency in wavenumbers, then, is 2,886 wavenumbers for HCl. These are the common units that are used to talk about the fundamental vibrational frequencies of molecules. And these are the common numbers used to discuss infrared spectra of molecules.
This unit of a wavenumber. It is a non-SI unit.
In other words, what you will often see is an infrared spectrum intensity at the photodiode, now, versus wave number. And right at 2,886 wavenumbers, that is the fundamental frequency in wavenumbers.
That is where the molecule makes the transition from v equal zero to v equal one. Now, the question is, what determines the frequency of the vibration of a molecule?
Well, what determines the frequency of that vibration, and here, I have now gone back to frequency because I am writing it in terms of some other parameters, are two parameters. One is the force constant k, and the other is the mass. What I have here is the reduced mass. I will explain that to you in just a moment, but this is essentially the mass. The frequency is given by one over 2pi times the square root of this k, the force constant over the mass.
sqrt(k / m)** That force constant, k, is a measure of the stiffness of the spring.
Another words, if you have a very stiff spring, k is very large. That is you need a lot of force to pull that spring apart, a lot of force to compress that spring. A large k means you have a very stiff spring. If you have a small k, that means you have a very weak spring.
It does not take very much force to stretch that spring or to compress that spring. So k is a measure of the stiffness of the spring. It also tells us something about the shape of the interaction energy.
Another words, what k actually tells us here is kind of the width of this well.
Here is my interaction energy, potential well.
If you have a very narrow width, this is a large k.
If you have a very broad width, here, this is a small k.
So, k, the force constant, tells us about the shape, here, of this well. Very narrow well, large k. Very broad well, small k. Now, I think that maybe you have seen this before in 8.01. Have you done the harmonic oscillator yet? No?
Yes? Some yes.
Some no. Mostly no, it sounds to me.
This expression, here, for the frequency, you are going to see in 8.01 some time.
It comes from the harmonic oscillator model.
You will remember that you have seen this before.
Those of you who have know what I am talking about.
Those of you who have not, just remember you are going to see this very soon once again. Now, the other parameter here that is important is this mu here, this reduced mass.
What the reduced mass is, is essentially the mass.
Now, if you had already done this in 8.01, what you are going to look at is a mass on the end of a spring attached to a wall. There will be a wall, then you will see a spring, and then there will be some mass m. When you do this problem as a harmonic oscillator, you are going to write nu here as k over this mass right there. But, in this example, this mass here is the only body that is moving.
Your wall is still. But, in the case of a molecule, both atoms are moving. What mass do we use?
Well, the fact that both are moving means we have a two body problem. And we are going to have to change the coordinate system to take into account that both masses are moving. We can do that easily.
And doing that easily involves using something called the reduced mass. The reduced mass takes a two body problem and reduces it to a single body, where the single body is this fictitious body with a mass given by the reduced mass. It is exact.
I did not make any approximations here.
But the bottom line is that this reduced mass is simply the mass of one atom, times the mass of the other, divided by the sum of the two masses.
And sometimes a useful approximation is when one of the masses is much lower than the other mass, so when m1 here is smaller than m2. Well, you can see in that case, if m1 is very small compared to m2, we are going to treat it like a zero. And then these two m's are going to cancel. You can see the reduced mass is roughly equivalent to the mass of the smaller body.
Sometimes I will ask you to use that approximation.
Other times, I will ask you to calculate the reduced mass. Let's look at some frequencies for some molecules, here.
I have an array of molecules. All of them roughly have the same force constant. I am going to look at the mass dependence on the fundamental frequency.
I have got the frequencies written both in terms of wave numbers and Hertz. The first thing I want you to notice is the fundamental frequency for hydrogen, 4,159 wave numbers. That is the highest vibrational frequency that we know about. Why is it so high?
Well, because those two hydrogens are so light, and the mass is in the denominator of that fundamental frequency. So if it is in the denominator, it is small, and the fundamental frequency is going to be high. Then look at these molecules, HCl, HBr, HI. In this case here, it is all a hydrogen bonded to a halogen.
For the most part, the reduced mass of these three molecules is that of hydrogen. If that is of hydrogen, since that is in the denominator for the expression for the frequency, well, the frequencies are going to be high in general. And they are, 2,886, 2,559. Not as high as molecular hydrogen, but still pretty high. And then we look at these three molecules, chlorine, bromine, iodine.
Both particles very heavy. The reduced mass is large.
Look at what happened to the frequency here.
The frequency really has gone down much lower than it was for HCl, HBr or HI. So, that is one parameter.
The other parameter, of course, is the force constant. Here are a set of molecules that have all roughly the same reduced mass, fluorine, oxygen, NO.
And I have written down their frequencies, both in wave numbers and Hertz. For molecular fluorine, which has a single bond, that frequency here is pretty low, 892 wave numbers. It is pretty low.
This is a single bond, which is easy to stretch.
It is not very stiff. It is a loose bond, so to speak. But now, look at oxygen and NO.
They have double bonds, which are stiffer.
If they are stiffer, k is larger, the fundamental frequency is higher.
Then, we look at CO and nitrogen.
They have triple bonds, which are even stiffer.
Stiffer bonds, larger k, higher fundamental frequency, that are those two parameters that dictate the frequency of vibration. We will do rotation on Wednesday. See you then.
Free Downloads
Video
- iTunes U (MP4 - 116MB)
- Internet Archive (MP4 - 206MB)
Audio
- iTunes U (MP3 - 12MB)
Caption
- English-US (SRT)