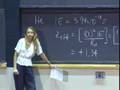Flash and JavaScript are required for this feature.
Download the video from iTunes U or the Internet Archive.
Topics covered: Periodic Trends in Elemental Properties
Instructors/speakers: Prof. Sylvia Ceyer

Lecture 10: Periodic Trends...
Related Resources
The following content is provided by MIT OpenCourseWare under a Creative Commons license.
Additional information about our license and MIT OpenCourseWare in general is available at ocw.mit.edu.
We were talking about the energies of the various states in a multi-electron atom. And the question is, how do we know what these energies are, experimentally? And the technique that we use to know those energies is something called photoelectron spectroscopy. In principle, it is the same kind of spectroscopy as when we talked about photoemission from a solid, the photoelectron effect explained by Einstein. And, by the way, I put on the website an article that is really very clear about what Einstein's contributions were.
In particular, in explaining the photoelectric effect. And it has some sociology in it, too. It is a really easy-to-read article. I encourage you to take a look at it as a study break sometime. Anyway.
The idea here is the same, the photoelectron spectroscopy off of atoms and molecules. It is the same thing as the photoelectron emission from a solid, in that you send in a photon and that causes an electron to be ejected.
But, unlike the solid where we essentially had just one state at a particular energy, in an atom or a molecule, we have many different states with many different energies.
And what we typically do, then, is we send in a photon that will be able to ionize all of those electrons.
That is, it is a photon with enough energy to rip off even the most strongly bound electron.
And so, that photon is typically an X-ray photon.
For example, we take this unsuspecting neon atom, and we bring in an X-ray photon and make neon plus.
And one of the electrons that can come off is, of course, the 2p electron, here, so that the ion configuration is 1s 2 2s 2 2p 5. And then, of course, another possible electron to be pulled off is that 2s electron.
The electron configuration is 1s 2 2s 1 2p 6.
And then, finally, if the photon has enough energy, we can pull off the 1s electron, to make the 1s 1 2s 2 configuration, 2s 2p6 configuration.
And what we then do in this technique is we measure the kinetic energies of all the electrons that come off.
We measure the kinetic energies and disperse them so that we get a plot of the number of electrons with a particular kinetic energy. The plot is what I show you here, number of electrons versus the kinetic energy.
And what you see is that we have three kinetic energies that show up. We have electrons with three different kinetic energies. We have three different kinetic energies because in neon, we had three states with different energies. So, for example, this energy, 384 eV, that corresponds to the electrons that were pulled off, electrons that were in that 1s state. That is the most strongly bound state. For example, if this is the energy of our incident photon, and then this is the ionization energy, which is going to be largest for the 1s electron because it is most strongly bound, well, then, the energy difference between the incident energy and that ionization energy is the kinetic energy of the electron. That is the leftover energy that goes into the kinetic energy of the electron.
That is going to be smallest because that 1s electron is most strongly bound. Then we have this kinetic energy here, electrons with that kinetic energy were electrons sitting in the 2s state. That has higher kinetic energy because they are less strongly bound.
This is the energy of the incident photon.
This is the ionization energy of that 2s electron.
That leftover energy, then, is that kinetic energy.
And then, finally, this is the feature that represents the electrons in the 2p state.
They are least strongly bound. If this is the incident energy, this is that ionization energy for the 2p state.
All of this energy is leftover to go into kinetic energy of that 2p electron moving away from the atom.
And so, in general, as the kinetic energy goes up, the binding energy here is getting less and less negative.
It is getting weaker and weaker.
Or, in other words, as the kinetic energy goes up, the binding energy is getting more negative in this direction.
Very quickly, if you look at a photoelectron spectrum and you see three lines like this, you know you have three states at three different energies.
And you always know that the highest kinetic energy means the least strongly bound electron, the lowest kinetic energy means the most strongly bound electron.
And then, we can use these results to actually calculate the binding energies of the electrons by conservation of energy. And this you have to know for the exam. Incident energy is that ionization energy or the work function, in the case of the solid, plus the kinetic energy. Or, I can turn this around.
I can solve for the ionization energy.
That will be the incident energy, which is right here.
This is the energy of that X-ray photon, 1,253 eV, minus the kinetic energies that we actually measure, which are going to give us those ionization energies for each one of the electrons in their corresponding state.
And then, once we have those ionization energies, it is easy to turn it into a binding energy because that ionization energy is equal to minus that binding energy.
For example, since the ionization energy, the 1s electron, is 870 eV, the binding energy of that 1s electron is minus eV.
And I also want you to notice just how much more strongly bound that electron in the 1s state is compared to the electrons in the n equals 2 shell.
That is because that n equals 1 shell is much closer in to the nucleus, where that attractive interaction is much stronger.
And, therefore, this electron is much more strongly bound than those electrons in the n equals 2 state. Of course, 2s is lower in energy than 2p, and that is important.
This is because in the 2s state, you have a finite probability of being really close to the nucleus.
That is what makes that 2s a little bit lower in energy than the 2p. Now, what we are going to do is I am going to start lecturing about trends in the Periodic Table, and this is where the questions come for your section.
Oh, okay. Do you have any extra paper?
Not much. Well, the way you were going to be able to answer a question for your recitation was by making a paper airplane and launching it to the blackboard after I say launch. And the first paper airplane to hit the blackboard, well, you will have the opportunity to answer the question.
Your recitation will have that opportunity to answer the question. And we will keep score.
Now, we are kind of running out of extra paper.
You can take the one page that asks for the seating plan that you will not need again, as a piece of paper to make an airplane. Do you have any other paper?
Here are a couple of extra pages.
Also, if you need some, there are some paper airplanes up here. You are welcome to take those.
Okay. Some of you are more strategically placed than others, and so you may want to designate someone in your recitation section as a launcher and send them down here so that everybody gets an equal chance.
That is fine with me. And, as I said, if anybody wants a paper airplane.
Oh, also the other thing, write your recitation section number on the airplane so that I can identify which airplane and what section it comes from. And they have to be airplanes.
They cannot be wads. And here are some extra airplanes if you want.
You can take some and pass them back if you want.
While you are doing that, I am going to start lecturing here about trends in the Periodic Table.
And, of course, it is these electron configurations that allow us to understand the trends in the Periodic Table. But you should realize that the Periodic Table was initially put together by Mendeleev and others in 1850-1870. And it was put together on the basis of the similarity of the chemical and physical properties of the elements. For example, lithium, sodium, potassium, they were put in the same column because those elements are all soft, malleable elements that were very reactive.
Helium, neon, argon were put in the same column because they are all atoms that are very unreactive.
And, of course, today, we understand why their properties are what they are. And we understand it on the basis of the electron configurations.
Lithium, sodium, potassium, we have this extra valence electron in the s state that makes it very reactive.
Helium, neon, argon are very unreactive because they have this closed shell, this inner gas configuration. But really, by the late 1800s and early 1900s, the similar chemical properties, as you go down a column of the Periodic Table, was really very firmly believed to an extreme extent.
So strongly, that it was known that, and, of course, human beings ate salt and that the body had sodium ions in it and potassium ions in it, if that is the case, if you can consume sodium and potassium, why not a little lithium?
In 1925 or so, there was marketed a soft drink, and this soft drink wanted a lemon-lime flavor to it. To get that lemon-lime flavor they needed a little bit of citric acid, but that is not very soluble in water. To make it soluble you make it a salt. And, for whatever reason, they used lithium citrate. Why not?
Let's use some lithium citrate. And that soft drink was none other than 7-Up. Honestly.
And, in fact, the benefits of lithium were touted. Lithium was claimed to give the beverage healthful benefits, an abundance of energy, enthusiasm, clear complexion, lustrous hair, lustrous eyes, shinning eyes, and you have got to drink this stuff.
It was the market for 25 years until the early 1950s, when the antipsychotic properties of lithium were beginning to be noticed, along with the severe side effects of lithium. And it was then taken off the market, or at least taken off the market with the citrate as the lithium salt in it. And it was really only another 20 years, 1970, before lithium was actually approved as a drug for the treatment of antipsychotic behavior. I don't know how many individuals suffered the side effects of lithium from drinking too much 7-Up, but there is a moral to this story. And the moral, of course, is that even though we are about to talk about the general trends along a column of a Periodic Table, don't put any element in your mouth just because it is in the same column of the Period Table as some element you do eat.
This is a for real story. One of the properties we are going to talk about is the ionization energy.
And we have already used the word ionization energy quite a lot, but let me just formalize the definitions here.
For example, we have a boron atom being ionized. The energy difference between the products and the reactions here is that ionization energy.
But this ionization energy is minus the binding energy of the 2p electron in boron. That is what that ionization energy is. We are ripping off the least strongly bound electron. And when we rip off the least strongly bound electron, we call that the first ionization energy. That is the energy to remove the electron from the highest occupied atomic orbital.
That is the same idea, the highest occupied atomic orbital, that is the same idea as the least strongly bound electron. However, most of the time, when we talk about ionization energy, we don't use the word first. If you see a list of ionization energies, if you see a table of ionization energies, they don't say first. That first is implied.
Now, of course, there are other kinds of ionization energies. For example, the second ionization energy. We can keep going here.
We can take boron plus, rip off the next least strongly bound electron to make boron plus 2.
That energy change is what we define as a second ionization energy. That second ionization energy is minus the binding energy of the 2s electron in boron plus.
And we can keep going.
There is a third ionization energy, taking another electron from boron plus 2 to boron plus 3.
And there is a fourth ionization energy, boron plus 3 to boron plus 4.
And a fifth ionization energy, boron plus 4, boron plus 5, all the way down.
And now we are the bare nucleus.
That is our definition of ionization energy, second, third, fourth, and fifth.
Now comes the first question, and the question is going to be on the side boards here. We have this reaction.
This is boron plus going to boron plus 2.
What we are doing here is removing a 2s electron.
[LAUGHTER] Remember about the fool aiming for their chemistry professor?
Look at the second reaction here, boron to boron plus.
Again, we are pulling off a 2s electron. And the question is, are these two energies equal? Now, the way we are going to answer it is after I get out of the way, I am going to say launch. You are going to launch.
And the first paper airplane to hit the blackboard, we are going to look at that recitation number and you are going to have a chance to answer.
Are you ready? Launch.
Recitation four. Are these two energies equal?
That is you.
Are these two energies equal? No.
Final answer? Yes, they are right.
One point for recitation four. These energies are not equal.
Why? Well, because this ionization energy is the second ionization energy. It is minus the binding energy of the 2s electron in boron plus.
This energy is the ionization energy of the 2s electron in boron.
It is minus the binding energy of the 2s electron in boron.
They are not equal. Next question.
Which one of these binding energies is greater?
Wait. I am getting out of the way.
Are you ready? In position.
Launch.
Recitation eight. Where are you, eight? You are over here.
Eight does not want to identify itself.
All right. Which one is greater, top or bottom? Top.
Final answer? Boy, you are right.
Top one is greater. Recitation eight.
It is greater because the effective charge in boron plus is larger than it is in boron. There are fewer electrons for shielding. The effective charge is larger.
That binding energy is greater. Or, that ionization energy here, the way I write it, is greater.
Fantastic. Now, we are going to look at some trends in the ionization energy along the Periodic Table.
The first question is, here is the Periodic Table, as we go across it, what happens to that ionization energy? Wait.
Are we in position? Ready?
Launch.
Recitation five. What happens to the ionization energy as you go across the Periodic Table?
Is that five? Is that your answer, five?
What happens to the ionization energy as you go across the Periodic Table from here to here?
It increases. You are right, recitation five. Here is the trend.
Here is row one, from hydrogen to helium.
Here is row two, from lithium to neon.
It increases. It increases because, as you go across the Periodic Table here, Z increases.
The nuclear charge increases. But, of course, there is another parameter here, and that is r.
But, as you go across the Periodic Table right here, you are putting electrons into the same shell, essentially. You are putting those electrons into shells that have, roughly speaking, the same distance of r from the nucleus.
And so the factor that wins out is Z, the effective charge here.
And the ionization energy as you go across then increases.
But you also see that there are some glitches.
You see that boron is a little bit lower energy than -- What did I want to say here? I'm sorry.
I am looking at it from the side.
Here is the electron configuration of beryllium.
Here it is, boron, Z equals 5.
What you see, here, is that in boron, you have to put that extra electron into the 2p state, which requires some more energy.
The bottom line is that the nuclear charge does not increase fast enough to compensate completely for the extra energy that you need to have to get to this 2p state.
Boron has a little bit lower ionization energy than beryllium. And to see another glitch, that other glitch is between nitrogen and oxygen.
Again, we can understand that glitch by the electron configuration. Here is nitrogen, Z equals 7. Here is oxygen, Z equals 8. And the bottom line here is that in oxygen, you have to put an electron in a state in which there already is an electron.
That is a repulsive interaction.
Again, the increase in the nuclear charge just is not large enough to fully compensate for that repulsive interaction.
We can understand those little glitches in terms of these electron configurations. And, in general, here, that ionization increases as we go across the Periodic Table. Here is the third row.
You also see there are glitches in the third row.
You see there is a glitch between magnesium and aluminum.
They are right underneath beryllium and boron.
A glitch between phosphorous and sulfur.
They are right underneath nitrogen and oxygen.
For the same reason, there is a glitch at those elements in the second row. Now, the next question.
As we go down a column of the Periodic Table, what happens to the ionization energy?
Wait. Ready?
Set? Launch.
Five. What happens to the ionization energy? What?
Decreases. You are right, recitation five. Why does it decrease?
It decreases because? Well, it decreases because you are putting electrons into shells that are farther and farther away from the nucleus. Yes, as you go down the column, Z increases. But it does not increase fast enough as r increases. Remember there are the two factors, Z and r. Although Z increases, you are putting the electrons into shells that are farther from the nucleus. Therefore, that attractive interaction does go down, and the ionization energy goes down. Great.
Now, we are going to talk about another property.
That other property is called the electron affinity.
For example, if I take chlorine and add an electron to it to make chlorine minus.
There is an energy change here. That energy change, delta E, is equal to minus kilojoules per mole.
That is, the fact that this energy change is negative, this tells you that the chlorine ion is more stable than the neutral atom.
Now, in terms of an equation, what is the definition here of the electron affinity? That is the next question.
Are you ready to launch? Launch.
Recitation eight. Where are you?
You are right here. No, you are right there.
Recitation eight, what is the definition of the electron affinity?
TAs cannot help. Energy required to add an electron, that is -- Recitation eight, you are right.
It is minus delta E. Fantastic.
This is the delta E for the reason.
The electron affinity is minus that quantity.
The electron affinity, then, of chlorine is kilojoules per mole. Now, unlike the ionization energy, the electron affinity can be positive or negative.
For example, if you try to stick an electron on a nitrogen atom to make N minus, delta E for that reaction is positive.
It is 7 kilojoules per mole. And so that electron is not going to stick onto the nitrogen.
The electron affinity in that case is negative.
It is minus 7 kilojoules per mole.
Again, you can understand that in terms of the electron configuration for the nitrogen. Here is the electron configuration for nitrogen. If you go and you want to add in another electron, like right there, there is a repulsive interaction.
And Z equals 7. In this case, that nuclear charge is not large enough to compensate for this repulsive interaction, so the electron affinity, here, is negative. Noble gases have negative electron affinities. You cannot add an electron to that. It is not stable.
Why? Because you are destroying, in effect, the electronic configuration of the rare gas.
With that, now we want to ask is how does the electron affinity change as you go across the periodic table?
That is our next question. Wait.
Okay. Ready?
Set? Launch.
Six, where are you? What happens to the electron affinity as you go across the Periodic Table, from left to right? Decreases.
Is that your final answer? Yes?
You are wrong. The electron affinity, as you go across, increases.
The halogens, fluorine, chlorine have very large electron affinities, because adding an electron gives you that noble gas configuration.
The electron affinity increases as you go across the Periodic Table. Now what happens to the electron affinity as we go down the Periodic Table?
You guys, do you have any paper airplanes?
We have to give you some. Are you okay?
That is our next question. What happens to the electron affinity as we go down the Periodic Table?
Ready? Set?
Launch.
Recitation four. What does it do?
Does it go down? Decreases.
It absolutely does.
The electron affinity, as you go down, decreases. It decreases because the nuclear charge, here, is increasing, but you are putting electrons in shells that are farther away from the nucleus. The overall effect is that that r dependence dictates, and the electron affinity goes down.
Let me do one more here. Or, maybe a couple more.
Now, what I want to ask is about the atomic radius.
And what I want to know is what happens to r as you go across the Periodic Table. I am getting out of the way.
Wait. Ready?
Set? I haven't said launch yet.
Ready? Set?
Go. Recitation one.
What happens to r? Decreases.
It decreases. It goes down.
This is important. It goes down, here, because Z is increasing. Z is increasing, and you are putting electrons into the same shell.
And so the same shell means the same distance from the nucleus.
Larger Z, the radius actually goes down, the size goes down.
And then, one more question. What happens as you go down the Periodic Table? I am going to get out of the way. Ready?
Set? Go.
One. It decreases?
What did you say? Increases.
It does increase because you are putting electrons into shells farther and farther away. What is the score, Christine? Fantastic.
Free Downloads
Video
- iTunes U (MP4 - 82MB)
- Internet Archive (MP4 - 146MB)
Audio
- iTunes U (MP3 - 8MB)
Caption
- English-US (SRT)