Flash and JavaScript are required for this feature.
Download the video from iTunes U or the Internet Archive.
Topics covered: Biochemistry
Instructor: Catherine Drennan, Elizabeth Vogel Taylor

Lecture 36: Biochemistry
Related Resources
Lecture Notes (PDF)
The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To make a donation or view additional materials from hundreds of MIT courses, visit MIT OpenCourseWare at ocw.mit.edu.
PROFESSOR: All right, so let's do our first clicker question. All right, let's just do 10 more seconds. Interesting.
So, in this problem you were given the maximum rate, Vmax, which, by the way, you calculated in class last time. And then said what will the substrate concentration be when you have half of that rate. And so, when you have half of the maximum rate, that's the definition of k m. So all you have to do is if a k m is given, you just write down that value. So that's the level of question you'll get on enzyme kinetics. All right, so that should be a good tie breaker, too.
All right, so in this little review and we talk about the material from the second half of the course, and why I think this material is really important and cool, and that's because it all represents the basic principles of how enzymes work. And as a biochemist, I recognize this material is really important. So that's what interests me particularly about it. And the point that I want to make in general is that when you're studying introductory material, sometimes it seems like you're never going to really need that material again, but that's because you haven't seen the connection between it in other topics. So, a lot of the things you've learned will be related to things that you're going to see you later on.
So I'm going to give you examples and review examples of how the material you've learned will allow you to understand an enzyme, for example.
So, we talked last time that one reason why you care about understanding how an enzyme works is because a lot of people want to inhibit enzymes. Well, why would you want to inhibit enzymes, and that's because inhibiting enzymes is very useful to treat a bunch of things. A number of you will, in the next week, I'm pretty sure, be interested in ways to inhibit enzymes to prevent headaches or to decrease headaches. And this is true of students and faculty during finals week.
And so, some of the treatments for headaches that a number of those pharmaceuticals are geared toward enzymes called prostaglandin synthesis, and they inhibit those enzyme from working and that gets rid of your headache. Arthritis, for example, is also treated by a lot of the same medications that treat headaches. Cancer, often chemotherapy or you're giving people inhibitors of enzymes to decrease tumor growth. And we talked last time about inhibiting HIV proteases as part of a treatment for HIV. So, understanding enzymes is very important for the pharmaceutical industry, and so that's one example of why you need to know about chemical equilibrium, why you need to know about acid based oxidation reduction transition metals, and also kinetics.
So, I'm going to give you a case study. You've seen some of these examples before, but we're going to put them all together now. And I try in every lecture, if I can, to mention vitamin B12, and here, we're going to tell you about a vitamin B12 dependent enzyme, and how knowing what you know now about these topics would allow you to understand how this enzyme works.
So, first, kinetics, this is easy, methionine synthase, it's an enzyme. So, what does this enzyme do? So this particular enzyme converts an amino acid called homocysteine to another amino acid, methionine. It also converts another vitamin, a B vitamin, the methyltetrahydrofolate form to tetrahydrofolate, so this folate is one your B vitamins. Why do you care? Well, methionine is needed for proper formation of brain and spinal column, and it's particularly important when women are pregnant that they have enough folic acid so that they can make enough methionine. If they do not have enough folic acid, then you can have babies born with neural tube defects. Also, as I mentioned before, inhibiting methionine synthase raises homocysteine levels, so you can't do this conversion, and that increases risk of heart disease that homocysteine is actually a really good indicator, high homocysteine levels of whether someone's going to have heart trouble or not.
This is also a target for chemotherapy, because you need tetrahydrofolate to do DNA biosynthesis, and tumor cells that are growing, tumors that are growing, need a lot of DNA biosynthesis. So if you inhibit this enzyme, you can inhibit DNA biosynthesis, so it's a potential chemotherapeutic target. There is some use of this in Europe. Actually there is a treatment that's given that's specifically supposed to inhibit methione synthase, it's not used so much in this country, but laughing gas will inhibit methione synthase. And so, in Europe some cancer patients are treated with laughing gas as a way to decrease their tumors. I'm not sure it's the most effective therapy, but they're at least pretty happy with it. So that's the counterpart.
So, this is an important enzyme. And enzymes, of course, as we know are catalysts and they catalyze reactions, so tell me what you know about catalysts. Which is the following statements about catalysts are true? OK, let's take 10 more seconds. Very good.
All right, so going back to the notes, statement 2 is true. So, catalysts work by lowering the activation energy barrier for both the forward and the reverse reaction. So both rates would be changed. And also, catalysts are not going to affect the thermodynamics, they're not going to shift the equilibrium toward products. What's something that you could use to shift an equilibrium, say, to products or reactants. Temperature, yeah.
So, just a little review of kinetics. Again, a catalyst works by lowering the activation energy barrier, or another way to say that is by stabilizing a transition state, so it's going to affect the activation energy for the forward reaction. And for the reverse reaction, it's going to decrease both of them by the same amount. So if you know how much one is decreased, you also know how much the other is decreased. And we also know that it doesn't affect the thermodynamics. So, does it change the equilibrium constant? No, it doesn't change the equilibrium constant.
All right, so a little review of kinetics. So now let's talk about transition metals. So this particular enzyme needs 2 different kinds of transition metals. It needs cobalt in the form of vitamin B12 and it also needs zinc. So we talked about methylcobalamin, so cobalamin is another name for vitamin B12. And here we have a methyl ligand, c h 3 is a methyl ligand coordinated to the cobalt in the center, and we saw before that when you have a thing that forms attachments at more than one point, it's called a chelate, so the corn ring here is a chelating agent, and it's a tetradentate ligand because there are 4 points of attachment. So we can have monodentate, bidentate, tetradentate, hexadentate, and tell you the number of points of attachment. And again, this is a chelate, and what do you know about a chelate effect? I want someone to tell me what the chelate effect is, and there's a special bonus prize of a teeshirt for the best answer. Who wants to give it a try and tell me what the chelate effect is. Any volunteers? Bonus teeshirt. Who wants to give it a try. Not very brave.
STUDENT: [INAUDIBLE]
PROFESSOR: OK, so chelate, it's something that's going to be binding to a metal at multiple points of attachment. And what do we know about how stable or how favorable that kind of interaction is?
STUDENT: It makes it more stable because [INAUDIBLE].
PROFESSOR: Are you reading from your notes? Well, you know what, I didn't say that was not allowed, so congratulations.
Right, so chelates are unusually stable due to entropic affects. So it's an entropy affect. So if you have a metal that's free in solution, it's going to bind to a lot of water molecules, and often, metals like to bind to thick things. You see a lot of octahedral complexes. So if you have a metal bound to 6 waters, and you bring in a chelate that will blind with multiple points of attachment, such as EDTA, which binds at 6 points of attachment, that 1 molecule of EDTA will displace 6 water molecules. So that is going to be entropically favorable. 6 things floating around in solution have a lot more entropy than 1 thing floating around in solution. And this makes chelates very stable. And so, if you want to get -- if someone is poisoned with the metal and you want to get that metal out of your system, you give them a chelate such as EDTA, hospitals have EDTA. It's also good, as we learned, for cleaning bath tubs.
All right, so that's the chelate effect.
All right, so what about zinc. This enzyme has a zinc and it has zinc in the plus -- zinc plus 2 ion. So, if you know that, you can calculate a d count. And so again, we can go to our periodic table. What group is zinc in? 12. So what would be the d count? 10. So 12 minus 2 equals 10. So what would you think would be true about the color of this d 10 system? All right, so let's just take 10 more seconds.
All right, so it would be colorless. And so, all of the d orbitals are going to be full, and so there's no transition from one set of d orbitals to the other, and so it would be a colorless compound. And that is, in fact, true that it took years and years of studying methionine synthase before people realized that it had a zinc in it, no one knew that there was a zinc, because they couldn't see it. It didn't add a color, and so no one knew that a metal was there for a long time.
All right, so what about oxidation reduction? So, this enzyme does a lot of different oxidation reduction reactions. So, when it reacts with homocysteine to form methionine, and the vitamin B12, which is shown in a cartoon form down here, goes from a plus 3 state that has the methyl associated, it gives the methyl group, the c h 3 group, up to homocysteine to form methionine, and then you're in a plus 1 state of cobalt here. But this state can lose an electron, and you can have a what's called Co(II) form of the enzyme, which you need to put another electron in and reduce it to go back, and you also need to methylate it again. And the methyl group comes from s adenosylmethionine. So, we need to think about how we're going to reduce the vitamin to get it back in its primary turnover state.
so, we know the redox potentials for this. The standard reduction potential for vitamin B12 is minus 0 . 5 2 6 volts. The potential reduction potential for flavodoxin, which is the protein that gives it the 1 electron, and it's a flavin protein, which is another B vitamin. And that's minus 0 . 2 3 0 volts. So, what do we know about these? Tell me which is the better reducing agent. All right, let's just do 10 more seconds. Click in your responses.
OK. So, vitamin B12 is a better reducing agent. If you have a large negative number, then the reduced species is very reducing. So it wants to reduce other things and get oxidized itself. So, that's interesting. It seems like vitamin B12 should be reducing flavodoxin and not the other way around. So we can look at how unfavorable this process is, and we can calculate a standard potential for this, and we can use this equation where we have reduction minus oxidation and plug the values in, and so we have a minus 0 . 5 2 6 minus a minus of the floating potential, and that gives us a negative value for delta e. So is this reaction spontaneous? No, and that's because if you have a negative standard reduction potential, then you're going to have a positive value for delta g, so it will not be spontaneous.
And again, you can calculate the value for delta g, so we have minus n, number of moles of electrons, Faraday's constant times the difference in potential, and it's a one electron process, we put in Faraday's constant and the calculated value for delta e, and we get a positive 28 . 6. And as I mentioned before in class, the way this reaction goes is that at the same time an electron goes in, you also cleave s adenosylmethionine, which gives the methyl group to also return to the catalytic cycle, and that process is very favorable minus 37 . 6 kilojoules per mole. So it drives the unfavorable reaction.
So in the body you will have a favorable oxidation reductions and you'll have unfavorable ones.
All right, so what do you a cell that requires energy to catalyze a non-spontaneous reaction? So, that's an electrolitic cell. And a cell that catalyzes a spontaneous reaction? Galvanic, right.
So again, with oxidation reduction, it doesn't matter if you're talking about a battery, or if you're talking about a cellular process, all the same equations apply.
All right, what about acid based equilibrium. So in this particular -- in this catalytic cycle, we are converting homocysteine to methionine, and that chemistry involves acid base. So what is true about the reaction is that the protonation state of the substrate, homocysteine, matters. So, you can have a protonated homocysteine, and here's the structure of homocysteine, a deprotonated homocysteine where the sulfur does not have a proton anymore. So we have an s minus here. And this protonated form of homocysteine can be converted to methionine where there's a methyl group attached to the sulfur here.
So, we want to know at physiological p h 7 . 4, how much of the homocysteine is deprotonated? And here, if you're asking how much of something is in a protonated state, how much of something is in a deprotonated state, what you are asking is what is the p k a of homocysteine. So what is the p k a of homocysteine? That's what you need to know to figure out how much would be in the protonated state, how much would be in the deprotonated state. So here, the p k a is 10. So now, without doing any calculations, I want you to tell me what you expect about how much is protonated and how much is deprotonated at physiological p h if the p k a is 10. All right, let's just take 10 more seconds.
I hope that I mentioned that acid base will be on the final exam. So good thing we're reviewing it right now.
All right, so now let's look at the math. So if you're given a p k a and p h and asked about a ratio of protonated to deprotonated it's OK to pull out your favorite Henderson Hasselbalch equation, which gives you a sense of the ratio, can predict the ratio, again it's approximation, but predict the ratio of protonated to deprotonated. H a, of course, being an abbreviation for protonated, a minus for deprotonated. And so we can calculate here that if you have a p h of 7 . 4, p k a of 10, you get a ratio of 400:1. So that's the math, but you can also just think about it in terms of what you know.
So let's just do a brief review. So the answer here is that free homocysteine is protonated and non-reactive at physiological p h. But let's just think about this question a little more. So this is not a figure in today's handout, but you've seen this a few times, so let's just look at it for a minute.
So, we talked a lot about acid based titrations. We talked about so here we have a weak acid tritrated with a strong base. In the beginning it's a weak acid problem, at the equivalence point it's a weak base problem, because we've added enough moles of our strong acid to convert all of our weak acid to its conjugate base. And in the middle when you've added half the number of the moles, you've converted half of your weak acid to its conjugate, then the p h is equal to the p k a at this point. So that would be right in the middle of this buffering region. Again, in a buffering region you have quite a bit -- you have quite a bit of your weak acid and quite a bit of a conjugate, so it can buffer. If strong acid is added, then it can be used up, if strong base is added, it can be used up without changing the p h very much. That's a buffer, so the p h curve is flat in here in that buffering region. And so, when you think about this, if you're at p h's that are below the p k a, then you're going to be more protonated, and p h is above the p k a, you'll be more deprotonated.
So let's look at the figure now that's in today's handout and think about this. So when the p h equals the p k a, you will have equal number of moles, of something that's protonated as deprotonated. And if you're at p h's above the p k a, you'll be more deprotonated. P h's below the p k a, you'll be more protonated. And in the particular example I gave, we have a p k a of 10, so at 7 . 4 we're at a p h that is below the p k a, and so here they'd be more protonated. So if you do the math it's 400:1. But even if you aren't doing any math, you should think about the fact that that would have more of the protonated form than the deprotonated form.
All right, but the enzyme has a problem, because only the deprotonated form is active. The p k a of the free homocysteine is 10, and you're at physiological p h, so this reaction doesn't seem like it should go. But again, it's an enzyme, and enzymes catalyze reactions. So they do things to make that reaction go faster. And what this particular enzyme does is it lowers the p k a of homocysteine. So when homocysteine is bound to the enzyme, it's p k a is no longer 10, but now its p k a is 6.
So, how does the enzyme do this? Well, that's where the zinc comes in. So, the zinc binds to the homocysteine and it acts as a Lewis acid as it binds to the homocysteine. So the homocysteine is your donor ligand, and you have your metal as the Lewis acid, your acceptor. And that alters the p k a, so it actually associates, zinc associates with this sulfur, and that changes the p k a.
So, I just wanted to mention again, I was having dinner with some of my faculty colleagues who teach organic chemistry here. We were interviewing another job candidate, and they looked at me and said, they're teaching organic this semester and they said we asked our class about p k a's, and they all insisted that they had never heard about p k a's in their freshman chemistry course. And I, of course, said, well, they did not take 511-1 then. So just remember, especially when it's Barbara Imperiali asking, did you hear about p k a's, the answer is? STUDENT: Yes.
PROFESSOR: Thank you.
OK, so just this week. So it alters the p k a here. So now what's our situation? Well, now at physiological p h, we have a p k a of 6, and so that gives us a very different ratio. Instead of 400:1, we have 1:25. So most of the homocysteine is deprotonated at physiological p h, which means that it can react. So, if we go back to this now, our p k a is now 6, and so physiological p h is now above that p k a. So when you're above the p k a, you should have more deprotonated than protonated. So that's how you can rationalize these things.
All right, now I have to ask, all right, we do not need a tie breaker. So at the end of the lecture, we will announce the winner. But let's first do chemical equilibrium.
Chemical equilibrium. So we didn't talk too much about this in chemical equilibrium, but enzymes can have alternate conformation, so the enzyme can change its shape during chemistry. And that those conformation of the enzyme can be in equilibrium. So the enzyme itself can be an equilibrium with different conformational states.
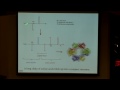
So here, there are a lot of states of the enzyme. It needs to react with homocysteine, it needs to react with methyltetrahydrofolate and with s adenosylmethionine. And when the structure was determined to this enzyme, here in green is the vitamin B12, and red is the methyl group. You see that the methyl group is pretty buried, you can barely see it, but yet it needs to interact with homocysteine to give it a methyl group, it needs to take a methyl group off methyltetrahydrofolate, it also needs to take a methyl group off s adenosylmethionine. But it doesn't seem like there's any room for any of them to actually get in there. So, this was a sign that there has to be some kind of change in the structure. So here's another picture of the structure. Here's the vitamin B12 in green, the methyl group in red, and there's this whole protein part up here that has to get out of the way to do the chemistry. And, in fact, we do know from experimental data that it does move so you can do the chemistry.
So that means that the enzyme has to have a number of different structures. It's modular. Here's the vitamin B12 binding domain, the vitamin B12, there's this region that have to move called the methyl cap. It needs to interact, the B12 here needs to interact with a folate domain, a homocysteine domain, and an activation domain. So you need to have at least these four different structures, and these four structures will be in equilibrium with each other. So you need to have a structure with folate, a binding domain on top of the red B12, you need to have homocysteine in yellow on top of the B12, you need to have a resting state where those helices are on top of the B12, and you need to have an activation state where the ado-meth domain is on top of the B12, and all of these states are going to be in equilibrium with each other, and they're going to be moving, which means that enzymes are dynamic, which means that chemistry is dynamic. Chemistry in the body is not in the solid state. Chemistry in the body is in solution.
Free Downloads
Free Streaming
Video
- iTunes U (MP4 - 68MB)
- Internet Archive (MP4 - 69MB)
Caption
- English-US (SRT)